October 3, 2022Management
AGC Begins Full-scale Study to Expand its Bio-CDMO Capability in Japan
― Selected for METI's "Developing biopharmaceutical manufacturing sites to strengthen vaccine production project" ―
As was the case for COVID-19, new types of vaccines utilizing biopharmaceutical technologies are being researched and developed around the world to combat new infectious diseases. Meanwhile in Japan there are limited production sites for biopharmaceuticals, including these new types of vaccines, and the development of production sites in Japan is an urgent issue.
Against this backdrop, the additional capabilities AGC is considering for the AGC Yokohama Technical Center consists of CDMO services for mRNA pharmaceuticals, cell and gene therapeutics, and protein based biopharmaceuticals made using mammalian cell cultures, which also can be applied for the manufacture of vaccines in the event of a pandemic. With the Bio-CDMO know-how that the Group has cultivated at its multiple sites, Japan, the U.S., and Europe, the Group aims to early expand its services in Japan, thereby adding to the domestic biopharmaceutical development and manufacturing capacities, and a more robust production network.
Notes
*1 METI: Japan’s Ministry of Economy, Trade and Industry
*2 Subsidy Project: METI has established a program to secure facilities that can produce biopharmaceuticals to meet the needs of companies during normal times and switch to vaccine production during emergencies, in order to prepare for future variants and new infectious diseases.
*3 CDMO: Contract Development & Manufacturing Organization. A company which is contracted on behalf of another company to serve product manufacturing as well as the development of manufacturing processes.
REFERENCE
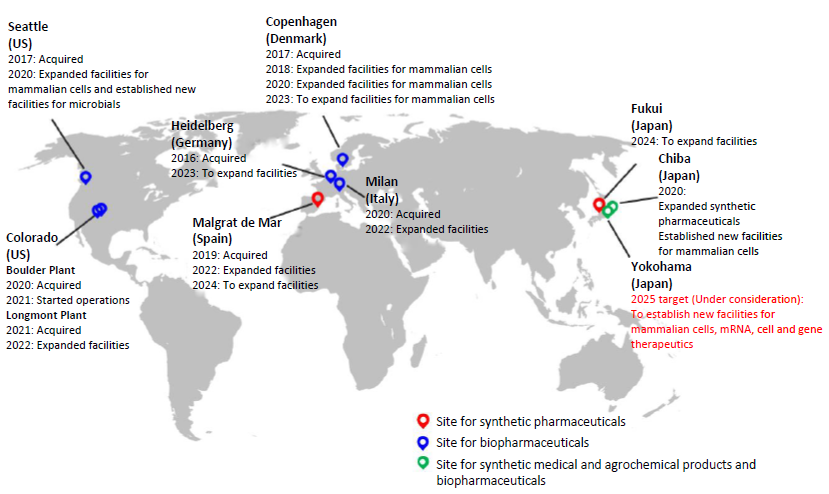
The AGC Group’s CDMO business locations
- Media inquiries
- Chikako Ogawa, General Manager, Corporate Communications & Investor Relations Division
AGC Inc. - Contact: Nakao
- TEL: +81-3-3218-5603
- Contact form