September 11, 2024Management
AGC and MEDINET Sign Strategic Partnership Agreement for Cell Therapy CDMO Business
-A New Initiative to Contribute to Enhancing Drug Discovery Capabilities in the Field of
Gene and Cell Therapy in Japan-
AGC (AGC Inc., Headquarters: Tokyo, President: Yoshinori Hirai), the parent company of AGC Biologics, a leading global Biopharmaceutical CDMO*1 and MEDINET (MEDINET Co., Ltd., Headquarters: Tokyo, President: Kanenao Kubushiro) have signed a strategic partnership agreement in the cell therapy CDMO business.
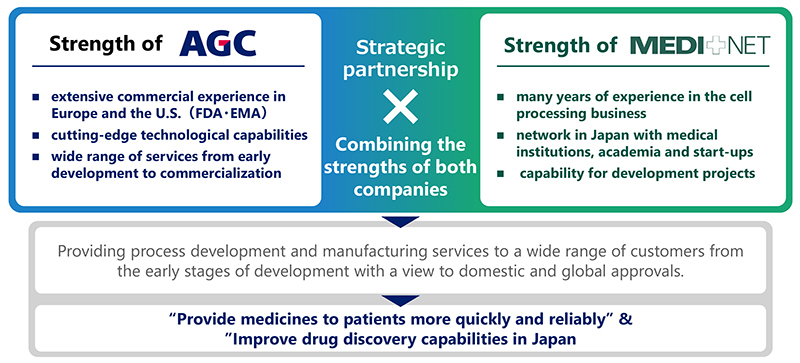
Strategic Partnership Combining the Strengths of AGC and MEDINET
In recent years, as the development and introduction of new modalities*2 such as gene and cell therapeutics has progressed worldwide, efforts to further improve Japan's drug discovery capabilities in this field have been discussed and promoted. As part of this effort, there is a need for global-level CDMOs in Japan that can provide process development and manufacturing services with a view to obtaining domestic and global regulatory approvals.
Under these circumstances, AGC and MEDINET have agreed to exchange and train manufacturing and quality human resources from both companies in order to complement MEDINET's capabilities with AGC Biologics' global network, and vice versa. Initially, AGC will send talents to MEDINET to build knowledge of this business in Japan by supporting MEDINET's operations, which has extensive experience in working with Japan based start-ups and academia, and to prepare for the launch of cell therapy CDMO services in Japan at the AGC Biologics’ Yokohama site, scheduled for 2026 (Part of the service will begin in 2025). The collaboration with AGC Biologics’ Sites, which have large-scale, global development and commercial stage manufacturing capabilities, will enable MEDINET to provide a wide range of services in its CDMO business and further expand its drug discovery and development business. The two companies will continue to discuss ways to work together effectively, including further expanding the scope of the collaboration.
AGC's strength lies in providing a wide range of services from early development to commercialization, based on its extensive commercial track record in Japan, Europe and the U.S. and its cutting-edge technological capabilities, while MEDINET's strength lies in its many years of experience in the cell processing business, its network with Japan based medical institutions, academia and start-ups, and its capability for development projects. By combining their respective strengths, the two companies aim to improve the quality of their services to customers and contribute to the enhancement of Japan's drug discovery capabilities.
Notes
*1 Contract Development & Manufacturing Organization. A company which is contracted on behalf of another company to serve product manufacturing as well as the development of manufacturing processes.
*2 Modality: A term used to describe a classification of methods and means of basic technology for drug discovery. e.g., antibody drugs, mRNA drugs.
About MEDINET
MEDINET has a cumulative experience of approximately 200,000 cases of cell processing in Japan over the past 30 years. Our business ranges from research and development of our own seeds in the field of regenerative medicine and other products to a CDMO business specializing in cell therapy, which is one of the few companies in Japan with GMP*-compliant capabilities.
*Manufacturing and quality control standards for drugs and drug products
About AGC Biologics
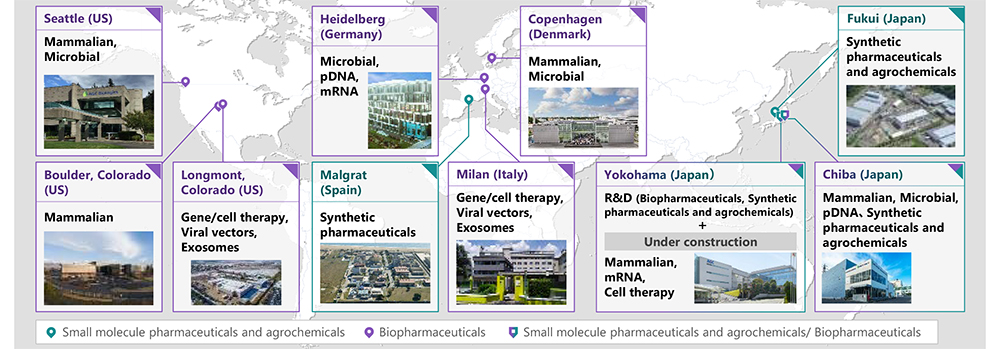
AGC Biologics is a part of the AGC Group, a world-leading manufacturer of glass, chemicals and other high-tech materials. AGC Biologics is a leading global biopharmaceutical Contract Development and Manufacturing Organization (CDMO) with a strong commitment to delivering the highest standard of service as we work side-by-side with our clients and partners, every step of the way. We provide world-class development and manufacture of mammalian and microbial-based therapeutic proteins, plasmid DNA (pDNA), messenger RNA (mRNA), viral vectors, and genetically engineered cells. Our global network spans the U.S., Europe, and Asia, with cGMP-compliant facilities in Seattle, Washington; Boulder and Longmont, Colorado; Copenhagen, Denmark; Heidelberg, Germany; Milan, Italy; and Chiba, Japan. We currently employ more than 2,500 Team Members worldwide. Our commitment to continuous innovation fosters the technical creativity to solve our clients’ most complex challenges, including specialization in fast-track projects and rare diseases. AGC Biologics is a part of AGC Inc.’s Life Science Business. The Life Science Business runs 10+ facilities focused on biopharmaceuticals, advanced therapies, small molecule active pharmaceutical ingredients, and agrochemicals. To learn more, visit www.agcbio.com. To learn more about AGC Biologics’ cell therapy CDMO services, visit www.agcbio.com/capabilities/cell-therapy. For more information on the company’s end-to-end global CDMO services in the U.S., Europe, and Japan visit www.agcbio.com.
|
The AGC Group's CDMO business sites and supported modalities |
|
|
Contact for inquiries related to this press release:
■ AGC Inc.
Chikako Ogawa, General Manager, Corporate Communications & Investor Relations Division
Contact: Nakao; Contact Form
■ MEDINET
Masami Ochiai, Corporate Communications & Investor Relations
Contact: Nosaka; Contact Form