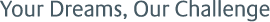September 24, 2024Management
CompleCure and AGC Partner to Develop Advanced Anti-Cancer Therapeutic Using Pioneering AMDC Technology
CompleCure (CompleCure Incorporated., Headquarters: Tokyo, President: Masayuki Tsuchiya), an innovative drug discovery start-up and AGC (AGC Inc., Headquarters: Tokyo; President: Yoshinori Hirai), the parent company of AGC Biologics, a leading global Biopharmaceutical Contract Development and Manufacturing Organization (CDMO) announced today that they have entered into a strategic service agreement. This partnership is focused on developing the manufacturing process for a novel anti-cancer therapeutic, which utilizes Antibody Mimetics Drug Conjugate (AMDC)*1 technology—a groundbreaking advancement in cancer treatment.
 AGC Biologics’ Chiba site
AGC Biologics’ Chiba site
AGC Biologics will apply its extensive expertise to develop the manufacturing process for the protein component of this new therapeutic at its cutting-edge facility in Chiba, Japan. AMDC technology represents a significant evolution of Antibody Drug Conjugate (ADC)*2 therapy, offering a promising new approach to cancer treatment by combining a mimetic protein*3 structure with a chemically synthesized drug specifically designed to target and destroy cancer cells.
The therapeutic, currently being developed by CompleCure, aims to revolutionize breast cancer treatment. It is expected to achieve complete remission with minimal side effects, thereby significantly improving patient outcomes. The protein component, which is the focus of AGC Biologics' efforts, features a complex tetrameric structure*4 that demands advanced technologies in microbial expression for successful process development and manufacturing—an area where AGC Biologics has demonstrated unparalleled expertise.
CompleCure selected AGC as its partner based on the company’s 30-year track record as a leading CDMO, its ability to rapidly propose innovative development solutions, and its flexible production capabilities. The two companies plan to continue manufacturing the protein component at AGC Biologics' Chiba site, ensuring a seamless transition to production for further steps once the process development is completed.
This collaboration marks a significant milestone in the path toward the commercialization of AMDC-based therapeutics. By combining AGC’s cutting-edge manufacturing capabilities with CompleCure's innovative therapeutic approach, the partnership aims to bring this promising treatment to market, ultimately contributing to the improvement of quality of life (QOL) for patients worldwide.
Notes
*1 AMDC (Antibody Mimetic Drug Conjugate): A term referring to the drug delivery methods and drugs using antibody mimetic molecules. This technology utilizes the strong binding activity of avidin-biotin complexes to easily create combinations of antibody mimetics that recognize various target molecules and drugs (payloads) that attack cancer cells, enabling the efficient discovery of highly effective treatments. The drug using AMDC technology consists of the following components: (1) A protein part that fuses a modified streptavidin variant, Cupid, which exhibits low immunogenicity and does not bind to biotin in the human body, with an antibody mimetic. (2) A chemical synthesis part that forms a non-covalent complex between Cupid and a payload conjugated to Psyche, a biotin variant with a high affinity for Cupid.
*2 ADC (Antibody-Drug Conjugate): Medications combining antibodies and drugs. ADCs are designed to deliver drugs directly to target cells by linking an antibody that selectively binds to antigens on the surface of specific cells, such as cancer cells, with a drug that attacks these cells. This design minimizes side effects and enhances therapeutic efficacy.
*3 Antibody Mimetics: Molecules that can specifically bind to antigens like antibodies, typically proteins with molecular weights of 3-20 kDa.
*4 Tetramer Structure: A molecule formed by binding of four monomer units through a modified streptavidin.
Reference
About CompleCure
CompleCure is a start-up developing innovative pharmaceuticals by utilizing cutting edge biotechnology. We own the intellectual property rights to the “Cupid-Psyche System,” utilizing the streptavidin and biotin’s high affinity and strong binding. Cupid-Psyche System is one of the outcomes of the “Molecularly Designed Antibody Project,” a cutting-edge R&D support program led by the Cabinet Office and involving Japan Bioindustry Association, the University of Tokyo, Osaka University, Chugai Pharmaceutical Co., Ltd. and Fujifilm Corporation.
The new breast cancer treatment for which AGC would develop manufacturing process under the announced strategic service agreement is the most advanced in development among the AMDC based therapeutics utilizing the Cupid-Psyche system. This therapeutics is being developed in cooperation with the University of Tokyo, Tohoku University, and Chiba University. We will open up new possibilities for fundamental treatment of cancer.
About AGC Biologics
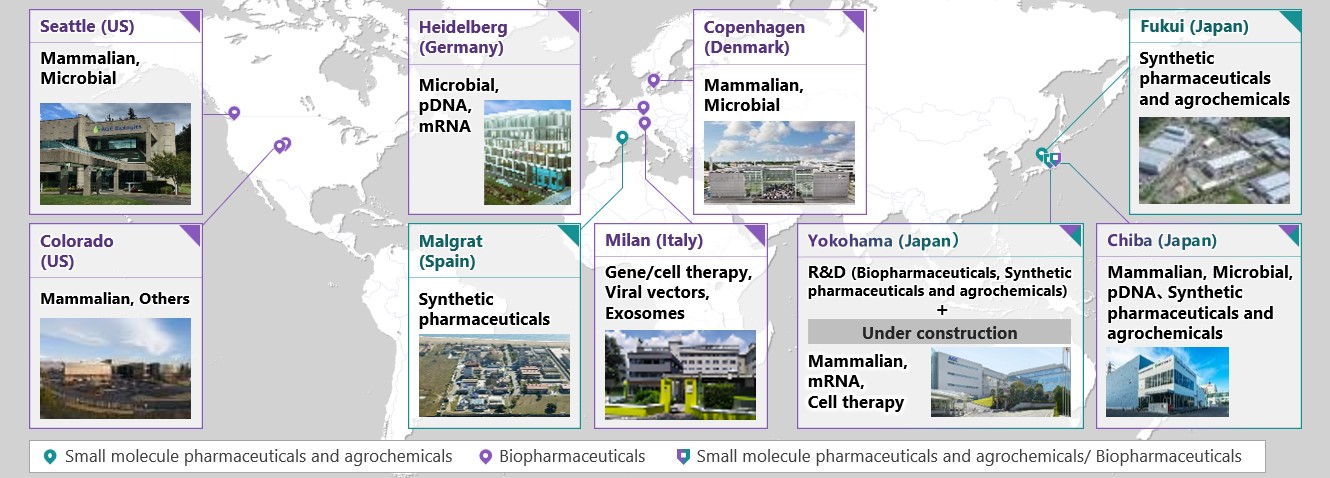
AGC Biologics is a part of the AGC Group, a world-leading manufacturer of glass, chemicals and other high-tech materials. AGC Biologics is a leading global biopharmaceutical Contract Development and Manufacturing Organization (CDMO) with a strong commitment to delivering the highest standard of service as we work side-by-side with our clients and partners, every step of the way. We provide world-class development and manufacture of mammalian and microbial-based therapeutic proteins, plasmid DNA (pDNA), messenger RNA (mRNA), viral vectors, and genetically engineered cells. Our global network spans the U.S., Europe, and Asia, with cGMP-compliant facilities in Seattle, Washington; Boulder and Longmont, Colorado; Copenhagen, Denmark; Heidelberg, Germany; Milan, Italy; and Chiba, Japan. We currently employ more than 2,500 Team Members worldwide. Our commitment to continuous innovation fosters the technical creativity to solve our clients’ most complex challenges, including specialization in fast-track projects and rare diseases. AGC Biologics is a part of AGC Inc.’s Life Science Business. The Life Science Business runs 10+ facilities focused on biopharmaceuticals, advanced therapies, small molecule active pharmaceutical ingredients, and agrochemicals. To learn more about AGC Biologics’ protein biologics manufacturing site in Chiba, Japan, visit www.agcbio.com/facilities/chiba. For more information on the company’s end-to-end global CDMO services in the U.S., Europe, and Japan visit www.agcbio.com.
The AGC Group's CDMO business sites
Contact for inquiries related to this press release:
■ CompleCure Incorporated.
Masayuki Tsuchiya, President
Contact: info "AT" complecure.com
* To send an e-mail message, please replace the "AT" in the address with an "@".
■ AGC Inc.
Chikako Ogawa, General Manager, Corporate Communications & Investor Relations Division
Contact: Nakao; TEL: +81-3-3218-5603; Contact Form