April 16, 2025Products
AGC's Pharmaceutical CDMO Division Won Four Awards at the CDMO Leadership Awards 2025
AGC Biologics, the biopharmaceutical CDMO*1 division and AGC Pharma Chemicals, the small molecule pharmaceutical*2 CDMO division of AGC (AGC Inc., Headquarters: Tokyo; President: Yoshinori Hirai), won a total of four awards at the CDMO Leadership Awards 2025.

The CDMO Leadership Awards 2025, sponsored by Outsourced Pharma and Life Science Connect, are decided through survey-based scoring using feedback from global biopharma and biotechnology organizations on the CDMOs. For 2025, out of a total of 210 companies, 289 respondents rated CDMOs with which they had actually done business in the previous 24 months.
As a result of this evaluation, AGC Biologics received the CDMO Leadership Awards both in the categories of “Biologics - Global*3” and “Cell & Gene - Global” and the Best In Class Award in the “Best Quality Management Systems. AGC Pharma Chemicals also received the Best In Class Award in the “Best Manufacturing Capabilities”.
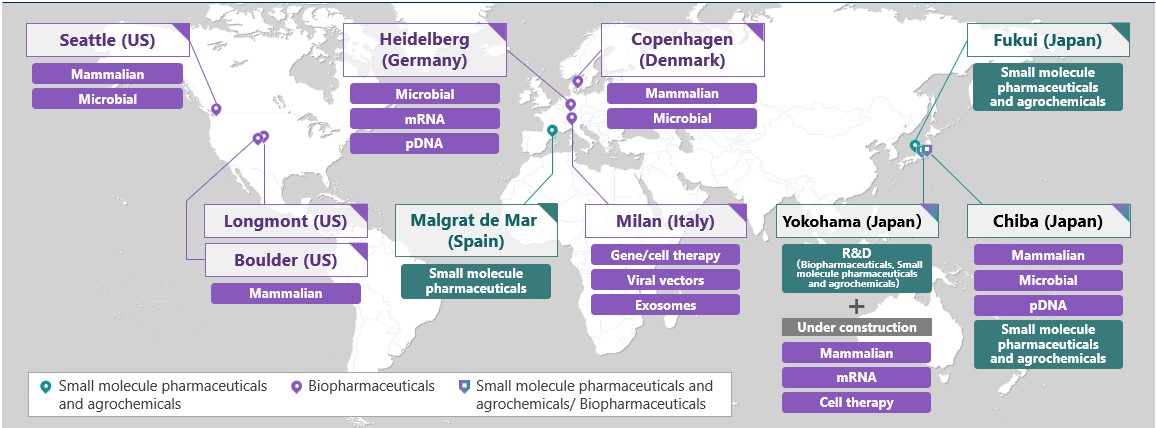
AGC Group's Life Science business provides cGMP*4-compliant CDMO services in three global regions: Japan, the U.S. and Europe. In addition to small molecule pharmaceuticals and agrochemicals based on organic synthesis technologies, the group focuses on biopharmaceuticals, for which demand is growing worldwide, and gene and cell therapies, a cutting-edge field. We will continue to contribute to a safe, comfortable and healthy life for people around the world by providing high quality services that meet the diverse needs of our customers.
Notes
|
*1 |
CDMO: Contract Development & Manufacturing Organization. A company which is contracted on behalf of another company to serve product manufacturing as well as the development of manufacturing processes. |
|
*2 |
small molecule pharmaceuticals: Pharmaceuticals manufactured through chemical synthesis. |
|
*3 |
Global: This award recognizes the top-tier CDMOs in each service area, divided into the following three geographic regions: Global (facilities both inside and outside of the U.S.), International (facilities outside the U.S. only) and North American (facilities in the U.S. only). |
|
*4 |
cGMP: Production and quality management standards for pharmaceuticals and quasi-drugs (current Good Manufacturing Practice) |
REFERENCE
Related press releases
AGC Biologics
AGC Biologics Honored for Excellence in Development and Manufacturing
AGC Pharma Chemicals
AGC Pharma Chemicals: Recognized as the Best Manufacturing Capabilities at the CDMO Leadership Awards 2025 - AGC Pharma Chemicals
|
Life science sites of the AGC Group |
|
|
- Media inquiries
- AGC Inc.
Corporate Communications & Investor Relations Division