June 26, 2025Others
AGC Launches Cell Therapy Process Development Services in Yokohama
— Transferring World-Class Technology from Its Milan Site to Meet Growing Market Needs —
AGC Inc. (Head Office: Tokyo; President: Yoshinori Hirai) , the parent company of AGC Biologics, will launch new process development (PD) services for cell therapy products at its Yokohama Technical Center (Tsurumi-ku, Yokohama) starting this July. By transferring advanced technologies from AGC Biologics’ Milan site*1, a world-leading CDMO*2 with a proven track record in gene and cell therapies, AGC has established capabilities to support a wide range of cell types, including T cells, NK cells, and mesenchymal stem cells. The launch of PD services precedes the start of services at a new CDMO facility to be established in Yokohama in 2026. By 2027, the Company plans*3 to offer full development and manufacturing services, including commercial production of mammalian-based protein biologics, cell therapies, and messenger RNA.

QC (Quality Control) Laboratory (left) and Cell Preparation Room (right) at our Cell Processing Facility
Cell therapies, a focus of regenerative medicine and cancer immunotherapy, are undergoing rapid global development and are expected to see significant market growth in the coming years. However, because these therapies involve working with living cells and tissues, the manufacturing process is highly complex, and there are currently only a limited number of CDMOs with a track record of commercial production. As the market grows, there is an increasing need for reliable CDMOs that can provide consistent support from research and development to commercialization in order to accelerate the practical application of new drugs.
Due to the nature of cell therapies, strict temperature control and rapid transportation are essential, making the geographical location of CDMO facilities a critical factor. To address this, AGC is establishing development and manufacturing capabilities not only in Europe and the U.S., but now also in Japan, aiming to deliver services to a broader range of customers and regions.
AGC Group’s Life Sciences Business aims to contribute to the realization of a society in which people can live safely, securely, comfortably and healthily, in other words, to improving “well-being”, by providing CDMO services for the development and manufacturing of pharmaceuticals and agrochemicals. Going forward, the AGC Group will continue to deliver globally consistent, high-quality services that meet the diverse needs of our customers, maximizing synergies across its network and creating value for pharmaceutical companies, patients, and society.
Notes
|
*1 |
AGC Biologics S.p.A. (100% subsidiary, headquartered in Italy) has a track record of nine commercial manufacturing approvals in the field of gene and cell therapies, where such experience remains limited globally. Additionally, it holds the top share as a CDMO in terms of the number of contract development and manufacturing projects for Ex Vivo gene therapy drugs (as of May 2025, based on AGC’s internal research). |
|
*2 |
CDMO: Contract Development and Manufacturing Organization. A company which is contracted on behalf of another company to provide product manufacturing services as well as the development of manufacturing processes. |
|
*3 |
Related release: AGC Decides to Expand its Biopharmaceutical CDMO Capability in Yokohama, Japan |
Reference
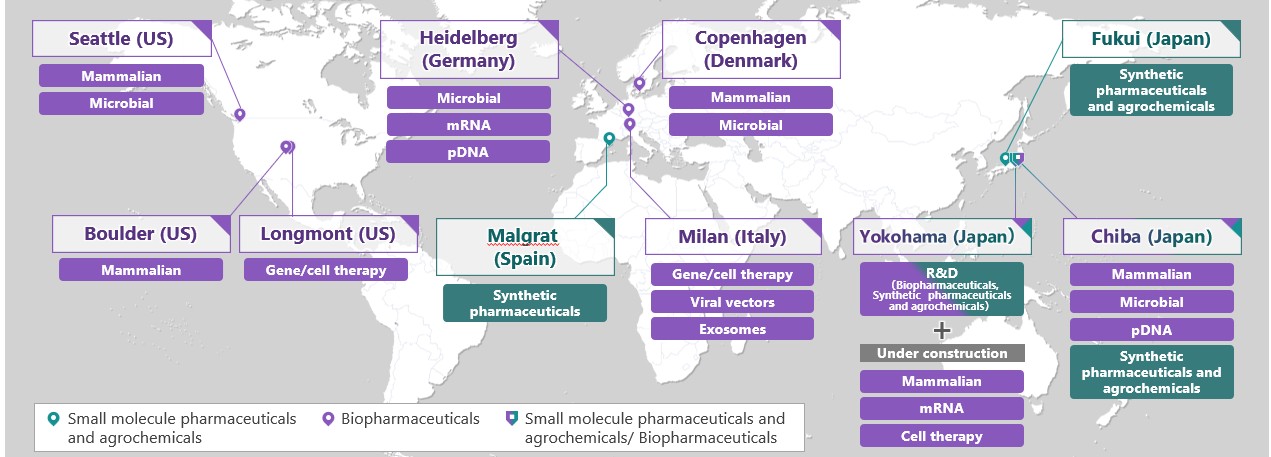
The AGC Group's CDMO business sites and supported modalities
- Media inquiries
- AGC Inc.
Corporate Communications & Investor Relations Division