January 19, 2026Others
AGC Biologics Announces U.S. and EU Marketing Authorization for Gene Therapy Waskyra™ Contract-Manufactured by Its Milan Facility
AGC Biologics S.p.A. (headquartered in Milan, Italy; hereinafter “AGC Biologics”), the biopharmaceutical CDMO*1 subsidiary of AGC Inc. (headquartered in Tokyo; President: Yoshinori Hirai), announces that the ex vivo gene therapy Waskyra™, which it contract-manufactured, has received marketing authorization from the U.S. Food and Drug Administration (FDA) and the European Commission. Developed by Fondazione Telethon*2, an Italian government-recognized non-profit biomedical research organization, Waskyra™ is indicated for Wiskott-Aldrich syndrome, a rare immune disorder. This approval enables patients to access a new treatment option.
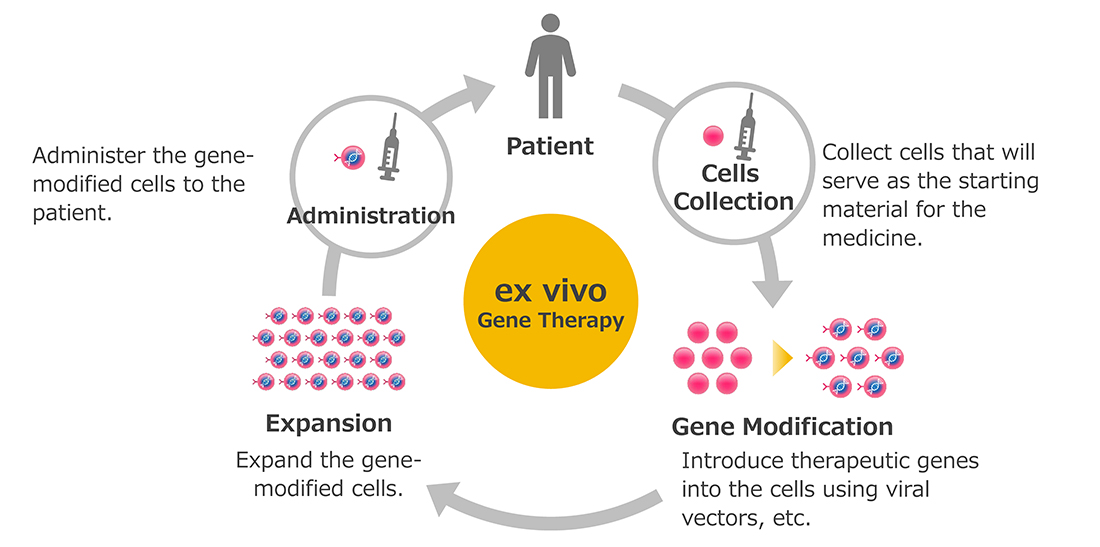
Wiskott-Aldrich syndrome is a rare immune disorder affecting approximately one in 250,000 live male births. Until now, treatment options have primarily consisted of symptomatic therapies and hematopoietic stem cell transplants from family donors. Waskyra™ is an ex vivo gene therapy that uses the patient’s own cells to correct the underlying genetic defect, avoiding donor dependency and the risk of rejection, while aiming for a curative outcome with minimal burden.
For this approval, AGC Biologics leveraged its global top-class experience in process development and contract manufacturing of gene and cell therapies*3, providing comprehensive support for lentiviral vector*4 production, patient-specific genetically modified cell manufacturing, and regulatory compliance, assisting with regulatory filings at every step. AGC Biologics will continue to provide support toward commercial manufacturing.
AGC Group’s medium-term management plan, AGC plus-2026, defines three social values. Among them, “Well-being” focuses on contributing to safe, secure, and healthy living by ensuring a stable supply of essential products and services for daily life and healthcare. Through its Life Science business, AGC continues to deliver globally unified, high-quality CDMO services for pharmaceuticals and agrochemicals, maximizing synergies across sites to benefit society.
Notes
|
*1 |
CDMO: Contract Development & Manufacturing Organization – a company that provides not only contract manufacturing of pharmaceuticals but also development of manufacturing processes. |
|
*2 |
Fondazione Telethon: An Italian government-recognized non-profit organization established in 1990. It promotes biomedical research aimed at developing treatments for rare and complex genetic diseases. |
|
*3 |
Based on commercial product portfolio. |
|
*4 |
Lentiviral vector: A vector is used to carry the target gene into a cell. A lentiviral vector is a type of vector that uses modified lentiviruses. |
- Media inquiries
- AGC Inc.
Corporate Communications & Investor Relations Division