February 19, 2026Others
AGC Biologics Secures GMP Certification from Brazil’s Anvisa
— Enables Supply to Brazil’s Largest Pharmaceutical Market —
AGC (AGC Inc., headquarters: Tokyo; President & CEO: Yoshinori Hirai) announced that its biopharmaceutical CDMO*1 subsidiary, AGC Biologics, Inc. (Headquarters: Washington State, USA; hereinafter “AGC Biologics”), has obtained Good Manufacturing Practice (GMP)*2 certification from Brazil’s National Health Surveillance Agency (Anvisa) for its Seattle site. With this certification, biopharmaceutical products manufactured at the Seattle facility can now be supplied to Brazil, the largest pharmaceutical market in South America.
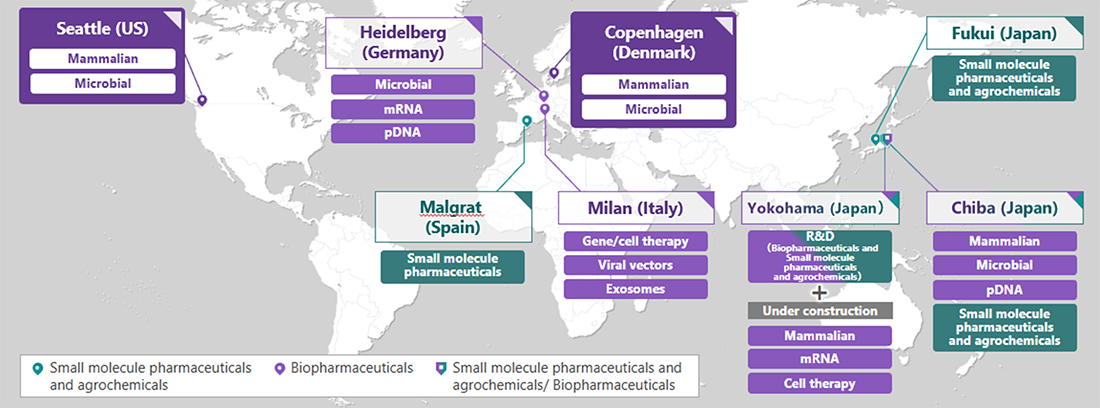
This marks the second AGC Group site to receive GMP certification from Anvisa, following AGC Biologics A/S (Headquarters: Copenhagen, Denmark). *3 Building on a globally integrated quality system and close technical collaboration among its sites, the AGC Group will continue to strengthen its cross-regional supply capabilities.

AGC Biologics Seattle Site, Washington, USA
The Seattle site has more than 30 years of experience in the development and manufacturing of biopharmaceuticals based on mammalian and microbial systems. In 2025, the site successfully passed all external audits, including customer audits, and achieved a 100% batch success rate, with all manufactured batches meeting quality standards—demonstrating its continued focus on quality excellence.
With the latest certification, the number of countries in which products manufactured at the Seattle site have obtained regulatory approval has reached more than 11.
Under its medium-term management plan, AGC plus-2026, the AGC Group has identified three areas of social value. As part of the “Well-being” pillar, the Group aims to contribute to safe, secure, and healthy living by ensuring the stable supply of products and services essential to daily life and healthcare. Through its Life Science business, AGC will continue to provide high-quality CDMO services for pharmaceutical and agricultural applications worldwide, maximizing synergies across its global sites and contributing to society.
Notes
|
*1 |
CDMO: Contract Development & Manufacturing Organization – a company that provides not only contract manufacturing of pharmaceuticals but also development of manufacturing processes. |
|
*2 |
GMP: Good Manufacturing Practice — Standards for manufacturing control and quality assurance of pharmaceuticals and quasi-drugs. |
|
*3 |
Anvisa-certified sites: see map below. |
Overview of AGC Biologics Seattle Site
|
Location |
Seattle, Washington, USA |
|
Primary Modalities |
Mammalian, Microbial |
|
Production Scale & Equipment |
Mammalian: 100 L–12,000 L; 3,000 L stainless-steel bioreactor, 2,000 L single-use bioreactor (SUB) |
- Media inquiries
- AGC Inc.
Corporate Communications & Investor Relations Division