March 6, 2018Management
AGC Enhances Biopharmaceutical Production Capacity at AGC Biologics
U.S. Berkeley Plant Installs New Bioreactor to Accelerate Scale-up Applications
AGC Asahi Glass (AGC), a world-leading manufacturer of glass, chemicals and high-tech materials, has enhanced the production capacity of biopharmaceuticals at AGC Biologics (*1), AGC’s CDMO (*2) subsidiary. With this capital investment, its Berkeley Plant (California, USA) has doubled in capacity and starts full operation this March 2018.
 Berkeley Plant Mammalian Cell Bioreactor
Berkeley Plant Mammalian Cell Bioreactor
AGC Biologics is a CDMO with mammalian and microbial facilities, providing a range of high value-added services from process development, scale-up to commercial production. The Berkeley Plant specializes in projects from initial development to phase II (*3) biopharmaceutical production and contracted development, offering a broad range of services from cell bank (*4) production, storage, cell culture, and purification. With the newly installed single-use (*5) 2,000L mammalian cell bioreactor, the Berkeley Plant will be able to meet diverse customer requirements from initial development to Phase II production and rapidly respond to scale-up production requirements. In addition, this will enable AGC to provide clients with a smooth consistent interface, and high quality services backed by proven track records at all of the locations worldwide.
Under its AGC plus management policy, the AGC Group has made a commitment to positioning its life-science business as one of its strategic initiatives. The AGC Group intends to continue making the necessary capital investment in its biopharmaceutical business, which is expected to exhibit significant growth in demand in the coming years. The AGC Group will continue to make a contribution to pharmaceutical companies, patients and society in general by maximizing synergies between each country and facility to enable a smooth and quick progression from development to commercial production.
Notes
*1 AGC has rebranded
its Bioscience Business under the name AGC Biologics from January 2018. Accordingly,
the legal entity name in the US operations, currently CMC Biologics, will be
changed to AGC Biologics in 2018.
*2 CDMO (Contract Development & Manufacturing Organization): A company that provides comprehensive services from development through manufacturing of biopharmaceuticals on a contract basis.
*3 Phase II: Phase II clinical trials
*4 Cell bank: Collection of cells of uniform composition
*5 Single-use: Bioreactors that uses a disposable vessel. It reduces the need for cleaning and antimicrobial processes and minimizes the risk of contamination.
REFERENCE
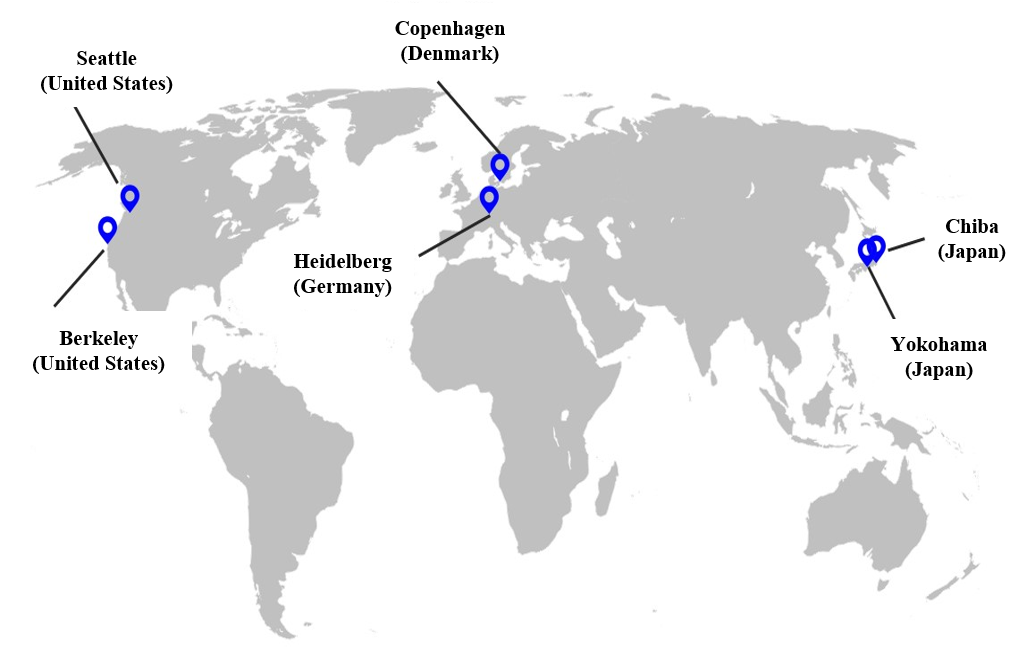
■AGC Group Bioscience Business Facilities
- Kazumi Tamaki, General Manager, Corporate Communications & Investor Relations Division
- Contact: Yuki Kitano
- TEL: +81-3-3218-5603
- E-mail: info-pr@agc.com