March 3, 2021Management
AGC Biologics to Expand Gene and Cell Therapy CDMO Capacity at its Milan Site
―Filling the growing demand for viral vector CDMO services―
 The Bresso Plant of AGC Biologics S.p.A.
The Bresso Plant of AGC Biologics S.p.A.
The AGC Group will continue to work with the pharmaceutical companies, contributing to the well-being of patients and society, by maximizing the synergy of each site so that the Group can provide its customers in each region with globally unified, high-quality services.
Notes:
*1 CDMO: Contract Development & Manufacturing Organization. A company which is contracted on behalf of another company to serve product manufacturing as well as the development of manufacturing processes.
*2 Viral vector: A vector is used to carry the target gene into a cell. Among vectors, viral vectors are those using modified cultured viruses.
*3 30% or higher annual growth: CAGR from 2020 to 2024. AGC estimate based on EvaluatePharma® World Preview 2017, Outlook to 2022 and other sources.
*4 GMP: Good Manufacturing Practice. A standard for the manufacture and quality management of pharmaceutical products.
*5 Suspension platform: A culture method used in the manufacture of viral vectors in which cells are free-floating in the culture media. It enables culturing on a larger scale compared with the adherent method, in which cells are attached to a substrate.
REFERENCE
| Established | 1996 |
| Headquarters | Milan, Italy |
| Sites | Milan plant/Bresso plant, Italy |
| Business activities | Gene/cell therapy drug discovery and CDMO |
| Shareholding ratio | AGC, 100% |
| Website | http://www.agcbio.com |
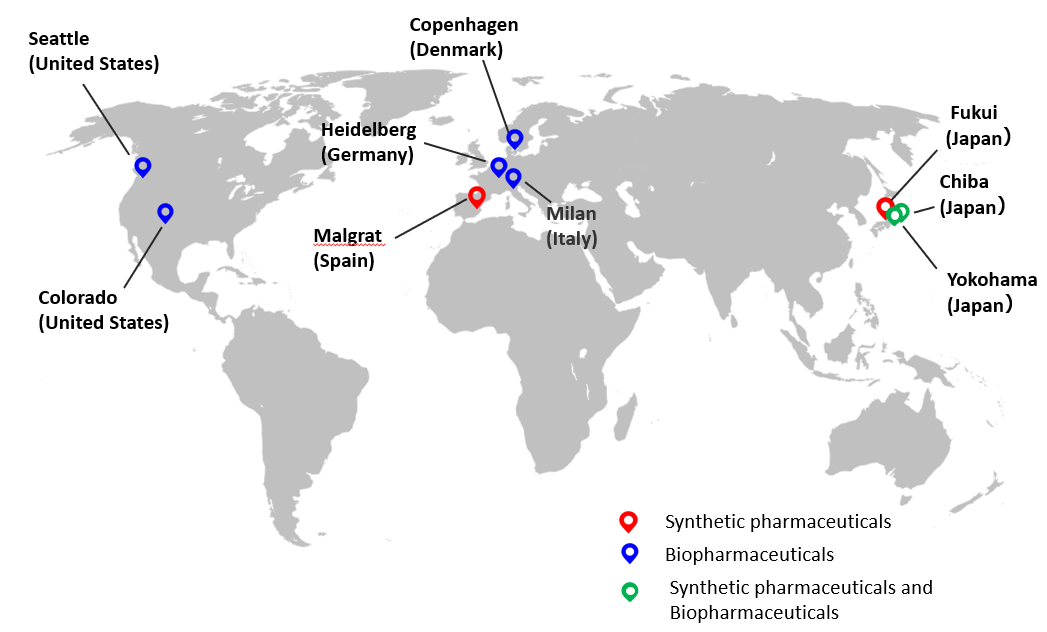
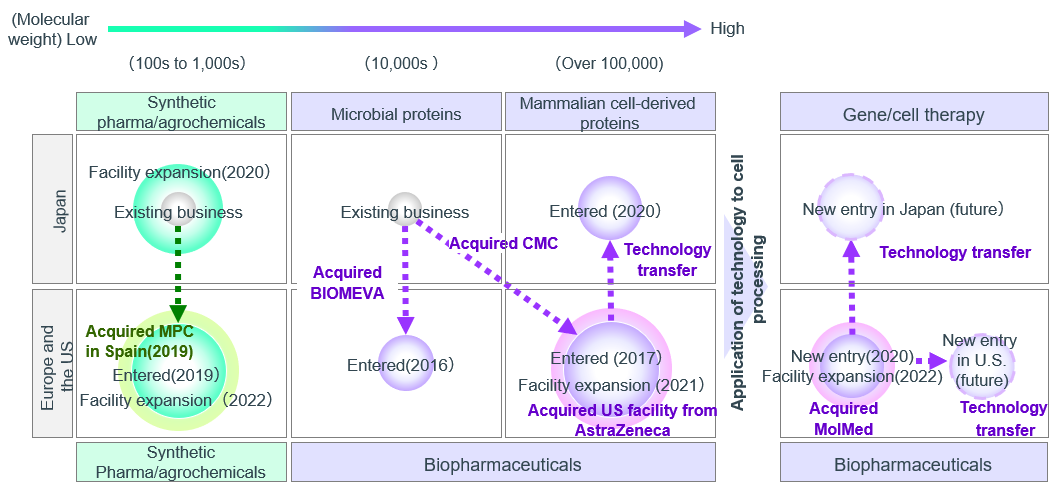
■AGC Group’s CDMO Business Sites
- MEDIA INQUIRIES
- Kazumi Tamaki, General Manager, Corporate Communications & Investor Relations Division
AGC Inc. - Contact: Tomoko Nakao
- TEL: +81-3-3218-5603
- E-mail: info-pr@agc.com