May 18, 2022Management
AGC Biologics Expands Cell and Gene Therapy CDMO Capacity at its U.S. site
- Responding to increased demand for viral vectors -
 Longmont site of AGC Biologics in the U.S.
Longmont site of AGC Biologics in the U.S.
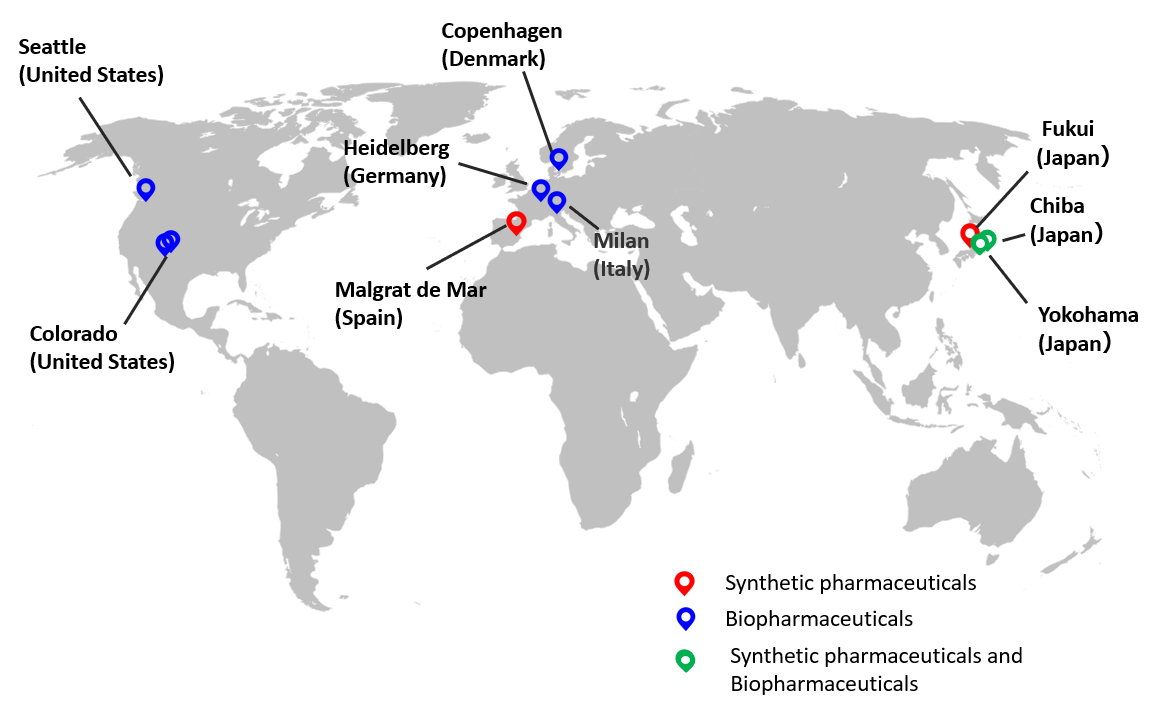
This expansion will include the introduction of a new suspension facility*5 suitable for the mass production of viral vectors, which is expected to grow rapidly in the field of gene and cell therapy. This expansion will be carried out with technical support from the Milan (Italy) site, which is also a cell and gene therapy site and has pioneered the introduction of floating culture facilities through the capacity expansion which was already announced. In collaboration with the Heidelberg (Germany) site, which is engaged in the contract manufacturing of plasmid DNA, a raw material for gene and cell therapy, we will continue to provide a one-stop global CDMO service from plasmids to gene and cell therapy drugs.
Notes
*1 CDMO: Contract Development & Manufacturing Organization. A company which is contracted on behalf of another company to provide product manufacturing services as well as the development of manufacturing processes.Synthetic pharmaceuticals: Pharmaceuticals manufactured through chemical synthesis, small molecule pharmaceuticals.
*2 Viral vector: A vector is used to carry the target gene into a cell. Among vectors, viral vectors are those using modified cultured viruses.
*3 Fiscal year of the Company: from January 1 to December 31
*4 30% or higher annual growth: CAGR from 2020 to 2024. AGC estimate based on EvaluatePharma® World Preview 2017, Outlook to 2022 and other sources.
*5 Suspension facility: A culture method used in the manufacture of viral vectors in which cells are free-floating in the culture media. It enables culturing on a larger scale compared with the adherent method, in which cells are attached to a substrate.
Reference
■ AGC Group’s CDMO business locations
- Media inquiries
- Chikako Ogawa, General Manager, Corporate Communications & Investor Relations Division
AGC Inc. - Contact: Nakao
- TEL: +81-3-3218-5603
- E-mail: info-pr@agc.com
- Personal information is handled in accordance with our Privacy Policy